Call For Collaborations
RePORT India Biorepository
RePORT India is a multi-institutional clinical and translational TB research consortium in India, jointly implemented by the Department of Biotechnology (DBT), India and the National Institutes of Health (NIH), USA. A total of ~3500 patients with TB and ~4500 of their household contacts were enrolled across all the Clinical Research Units (CRUs) in Phase I (2014 – 2019) of RePORT India. Phase II activities commenced in 2020 and is ongoing. The consortium strongly believes that some of the best research comes out of collaborative efforts and successful combinations of complementary skills and we welcome collaborations with all stakeholders. We are open to National and International collaborations for using our archived biospecimen to solve research questions in TB or to collaborate with us for new studies using the RePORT India resources.
Parent Protocol phase i
Phase I of RePORT India was initiated in 2013 in which seven Indian institutions collaborated with five Universities from the US to establish cohorts of persons with TB (PWTB) (Cohort A) and their household contacts (Cohort B). Initially, each Indian site had individual studies with unique objectives known as ‘Parent Protocol’.
Links to the parent protocols and the archived samples available to share with collaborators are attached. Archived samples from parent protocols may be obtained by contacting the site PIs and please reach out to RePORT India Administrative Coordinator for facilitation.
Common Protocol Phase I
In 2017, the RePORT India Common Protocol Phase I (CP Phase I) was initiated with an aim to collect specimens to create a central biorepository of Cohort A and Cohort B to make them available for biomarker researchers and collaborators to gain a better understanding of prognosis of TB disease and pathogenesis of progression from TB exposure to disease.
Links to the CP Phase I protocol and the archived samples available to share with collaborators are shared. Archived samples from CP Phase I may be obtained through RePORT India Concept Sheet process which is given at the end of the document.
Cross Consortium achievements of RePORT India in Phase I
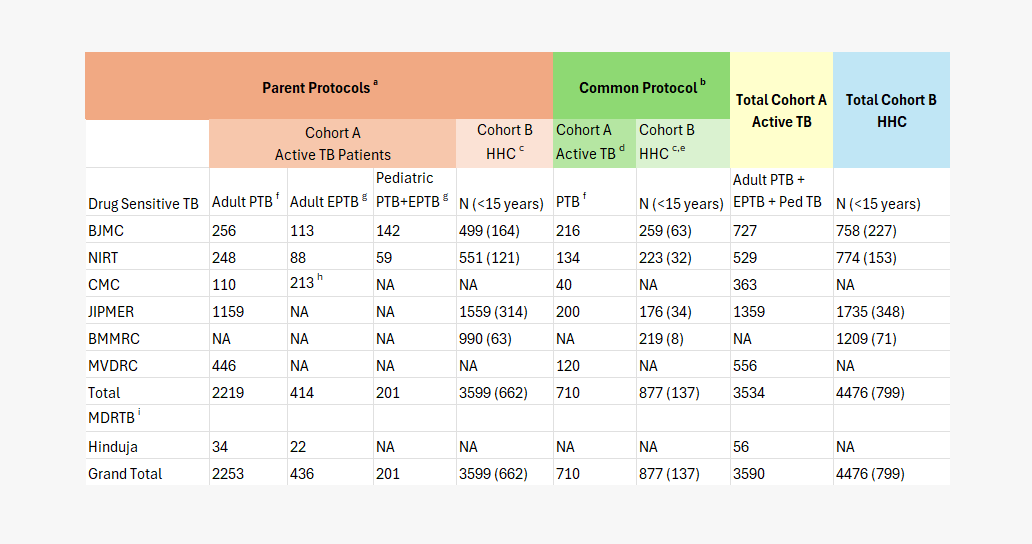
phase i enrollments (2013 – 2019)
aParent Protocols initiated in 2014; Follow-up ends 2019; bCMC Common Protocol Initiated 2017; Follow-up Ends 2020; cHHC-household contact of PTB; d99(14%) have completed 6m of flu in Common Protocol Cohort A; e0 have completed Common Protocol Cohort B follow up; fPTB-pulmonary TB; gEPTB=extrapulmonary TB; hCMC enrolled only adult TB meningitis patients; iMDRTB=multidrug resistant TB; Hinduja follow-up ends 2022
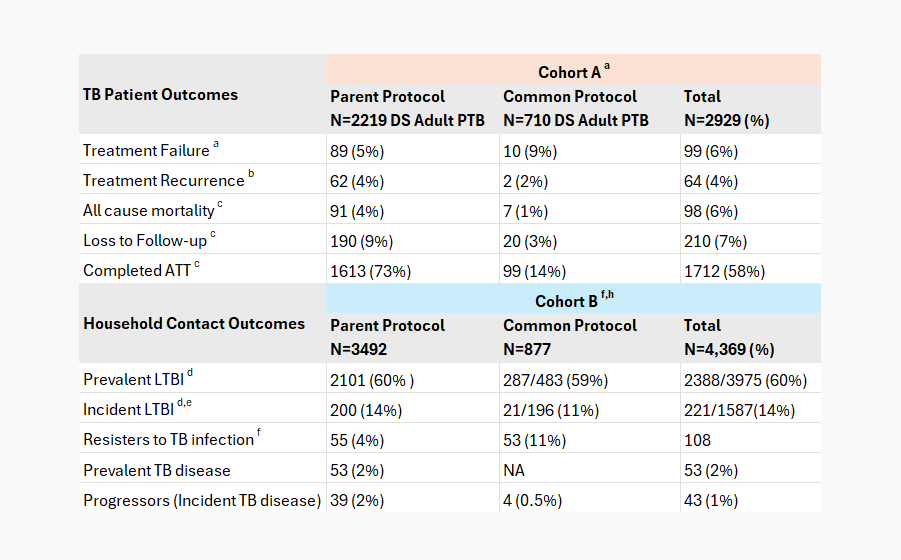
clinical & microbiological outcomes of phase i participants
(eptb & ped tb excluded)
PTB=Pulmonary TB; DS PTB-Drug-Susceptible Pulmonary TB; HHC-household contacts; LTBI=latent TB infection; adenominator includes those who failed treatment and those who completed anti-TB treatment; bdenominator includes only those who completed anti-TB treatment; cdenominator includes all drug-sensitive adult pulmonary TB cases; dTST or IGRA positive; denominator excludes prevalent LTBI; fThose who tested negative for both TST and IGRA over follow-ups; gdenominator excludes prevalent TB disease; hNone of cohort B have completed 24 months of follow up
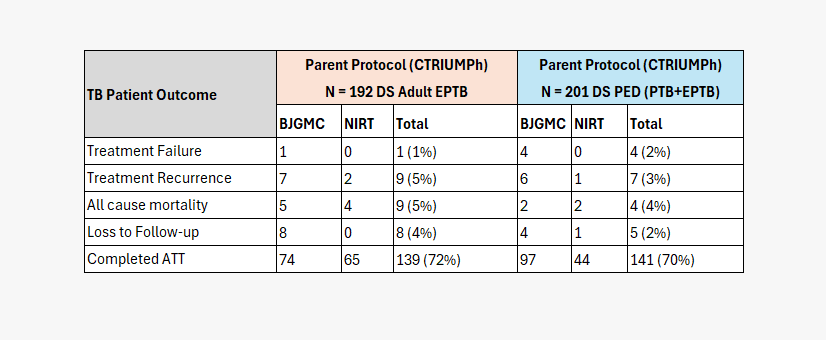
CLINICAL & MICROBIOLOGICAL OUTCOMES OF PHASE I PARTICIPANTS
(EPTB & PED TB)
Common Protocol Phase II
Phase II of CP was initiated with an aim to do extensive TB science using archived samples from Phase I as well as enrolling new study participants. A new presumptive TB cohort (Diagnostic Cohort) was added to the study in addition to cohort A and B. Please see the given link for CP Phase II protocol and the sample inventory of Diagnostic Cohort from which we are looking for novel diagnostic platforms. Cohort A and Cohort B components are still ongoing and will continue until Sept 2026. Samples form Diagnostic cohort may be obtained through RePORT India Concept Sheet process details of which is given at the end of the page.
Cohort, Biospecimen
General cohort description of the study participants of RePORT India is given below along with the common procedures utilized in collection and handling of biospecimen.
Procedure of biospecimen request for Common Protocol
Submission of concept sheet (CS) is required for all sample/data request from common protocol. Concept sheet and detailed procedure on sample request procedures are available below.
Once concept sheet is submitted the proposal will be reviewed by the consortium and a decision on data/sample sharing will be taken. The results will beintimated to the primary investigator applying the concept sheet. Studies involving Indian funding and samples processed within the country will ONLY need to have an approved concept sheet to utilize data/samples. If the study involves foreign collaboration, in addition to an approved concept sheet further screening will be doneby the Government of India through BioRAPP. Further details are provided in the data and sample sharing SOP given above.
Kindly contact RePORT India Administrative Coordinator for any further questions.