Concept Sheet & Publication Policy
Concept Sheet & Publication Policy
Version 2.1 (Nov 2018 approved)
The RePORT India Consortium was set up to address the threat of tuberculosis (TB) that affects the lives and well-being of people in India and across the globe and which poses an increased risk for persons living with HIV. A standardized concept sheet and publication policy is essential to ensure this main goal of the RePORT India Consortium is met, mainly through scientific collaboration and publication in scientific journals. Application of the policy will allow the RePORT India Consortium to track scientific concepts as they undergo various stages in preparation for publication; therefore, all investigators who wish to use and publish RePORT India data MUST follow the publication policy below.
A. CONCEPT SHEETS (CS)
Submission of concept sheet (CS) proposals is required for all proposed investigations involving analyses using existing data sets, the collection of new data (questionnaires, clinical and physical measures), and/or use or collection of laboratory specimens. Proposed RePORT India manuscripts based purely on individual site parent protocol data do not require prior CS approval by the RePORT India Executive Committee (EC), however this process may be used to facilitate specific PI approvals and the resulting manuscripts and abstracts should be reported per publication policy guidelines.
Investigators external to the RePORT India Consortium must work with a RePORT India liaison who is one of the RePORT investigators on the EC. To identify a potential RePORT India liaison, external investigators may contact the RePORT India Administrative Coordinator, Nancy Divya,
1. CONCEPT SHEET SUBMISSION AND REVIEW PROCESS
The CS submission, review, and approval process is described below:
1.
Submission of concept to RePORT Consortium
2.
Assignment of reviewers
3.
Circulating for review and comment
4.
Discussion
5.
Scientific Working Group vote
6.
Executive Committee (EC) vote
1.
Submission of concept to RePORT Consortium
Proposals must be submitted to the RePORT India Consortium electronically by emailing the RePORT India Coordinator, U.S. Secretariat, and Executive Committee Chairs using the Full Concept Sheet Submittal Format reportindiacoordinator@gmail.com. The RePORT India Coordinator will be responsible for ensuring that concept sheets are complete and appropriately processed. Any CS submitted with incomplete or outdated submittal forms will be returned to the author for correction before it is circulated for review. Before submission, it is recommended that concepts be discussed with appropriate scientific working group (WG) members, Principal Investigators and/or Study Coordinators to evaluate the science and assess the feasibility of the study. Investigators are requested to provide a timeframe for completion of proposed concepts. It may be difficult to determine the timeframe that involve collection of new specimens or data (i.e., new protocols). The timeframe will depend on the timing of protocol implementation and/or the availability of data and specimens. The investigator should provide a tentative but realistic timeline since this is tracked by the RePORT Consortium. When a CS is received by the RePORT India Consortium, the RePORT India Coordinator will fill out an internal checklist, appoint the scientific WG and assign individual reviewers. The process from time of concept submission (Step 1) to EC vote (Step 7) is expected to take approximately 4 weeks.
2.
Assignment of reviewers
Approximately 2-3 day period. The RePORT India Coordinator will work with the EC Chairs to assign a Scientific WG to the CS. To ensure that all concepts receive critical evaluation, the RePORT India Coordinator will then work with the chairs of the assigned Scientific WG to select specific reviewers from the below list, as appropriate. (See Appendix B for a list of potential reviewers.) The RePORT India Coordinator will confirm that each selected reviewer is willing and available to provide comments within the given timeline. They will also assign each CS a CS number to distinguish it from other concept submissions. The CS number will be used to track the project for all manuscripts and other projects that ensue.
A Primary Investigator Reviewer (PIR) is most often selected from scientific WG members, but reviewers can also be site PIs and other co-investigators with the appropriate knowledge and background suited to evaluate the proposed CS (assignments may be made on a rotating basis). Proposed PIRs with potential conflicts of interest (e.g., investigators from the site that is proposing the CS) should recuse themselves from the evaluation process. The PIR should review all posted comments before giving a final approval rating for a CS.
A Study Coordinator Reviewer (SCR) from among the RePORT India clinical sites, will be assigned to review a CS if it will require the collection of new data or new specimens, or if its implementation will impact clinic operations.
A Lab Reviewer (LR) will be assigned to review a CS if any new specimens will be collected from participants, if specimens will be withdrawn from the RePORT India Central Biorepository or if other laboratory expertise is required for appropriate review of the CS. The LR may serve as the PIR for laboratory based studies. Additional review may be given if the CS proposes the use of high-value specimens (see section C2b for details).
A Genetics Reviewer (GR) will be assigned to review a CS if DNA specimens are being requested from the RePORT India Central Biorepository, if the investigator intends to collect additional genetic materials, or create his/her own genetic materials from study specimens, or if any genetics analysis is to be done as part of the concept sheet.
3.
Circulating for review and comment
Approximately 1 week period. The RePORT India Coordinator will circulate the CS to the appointed reviewers via email and request comments during the specified review period. At the end of the review period, the PIR will circulate the results of the comments/discussion/review along with his/her ultimate recommendation for approval or rejection. PIRs should consider comments made by others, the significance of and need for the proposed investigation, whether the project overlaps with other ongoing or submitted proposals, and whether the science is relevant to RePORT India. If there is overlap with existing projects, then the PIR must indicate if the project should be combined with other related projects or revised to avoid overlap. The PIR will recommend if the CS should be approved as is, approved with comment, approved with major revision, corrected. If the CS was submitted by an external investigator, it is the responsibility of the PIR to assign a RePORT India collaborator to work with the investigator. The PIR will list the name of the suggested RePORT India collaborator on the CS Review form. The RePORT India Coordinator will then circulate the name of the assigned collaborator to the Scientific WG Chairs and EC Chairs.
4.
Discussion
Approximately 1 week period. After the review, the CS may be discussed at a WG meeting/call. In general, all CSs that require collection of new data or modifications to existing data collection instruments or methods (new or modified) should be discussed by the WG during a call or in-person meeting prior to WG vote. This additional discussion may be scientific (e.g., questions related to: is science relevant to RePORT India, should project be high priority, etc.), or operational (e.g., questions regarding implementation, overlap, etc.) in nature. Purely analytic or methodological CSs may go straight to WG vote if the PIR and WG chairs agree that further discussion is not needed.
5.
Scientific Working Group vote
Approximately 2-3 day period. Once the review of the CS has been completed, it will go to WG vote. A two thirds majority WG vote is required for the approval of new CSs. The WG can vote to approve without revisions, approve with revisions, suggest revisions subject to WG re-approval, or reject the CS. If a Scientific WG member is part of the project, he/she will abstain from voting.
6.
Executive Committee (EC) vote
Approximately 1 week period. Once the Scientific WG has approved a concept sheet, it will go to EC vote. A two-thirds majority EC vote is required for the approval of new CSs. In additional, RePORT India funders represented on the EC must approve before the CS can be implemented. The EC can vote to approve or reject CSs. CSs may be discussed at the EC meeting or submitted directly for vote by the EC at the discretion of the EC chairs. If an EC member is part of the project, he/she will abstain from voting. After the voting period, the RePORT India Coordinator will inform the submitting investigator of the outcome and will share reviewer comments (names removed).
CS review and discussion by the EC will be performed on an ad hoc basis. If numbers are deemed sufficient, a regular (quarterly or other) review schedule may be imposed.
2. SITE-SPECIFIC CONCEPT SHEETS
Site-specific CSs related to the Common Protocol are those that propose to utilize and/or collect Common Protocol data from one site only. Site-specific CSs need not go through the above process. However, they should inform the RePORT Indian Coordinator, U.S. Secretariat, and the EC Chairs by emailing reportindiacoordinator@gmail.com. This is for tracking purposes and to avoid duplication. Site-specific CSs do not require a PIR, SCR, LR or GR review, unless central RePORT India Consortium resources (e.g., funding, repository specimens, specific CHRD-SAS generated data sets, etc.) will be used. If RePORT India Consortium resources are requested, the CS should go through the approval process that is outlined in section 1 above but should use the Short CS Submittal Form. Site-specific concept sheets that do not utilize RePORT India Consortium resources can be shared with Scientific WG members at the discretion of the site PI, however is not required (e.g., may be done to solicit feedback). Approval of concepts that do not rely on consortium resources should comply with individual site procedures and policies. Site-specific CS publications will not be assigned co-authors from all sites and do not require funder approval. Studies involving multiple site parent protocols but not the Common Protocol need not go through the whole of this CS process, however they need to inform the RePORT India Coordinator, U.S. Secretariat and WG for tracking purposes and to avoid duplication of similar studies.
3. REVISED CONCEPT SHEETS
Investigators with a previously-approved CS may wish to amend a CS to request additional specimens and/or data. In this case, the original CS Submittal Form should be revised. The investigator should highlight all changes and additions in track changes. In all cases possible, the original PIR, SCR, LR and/or GR should be assigned to review the revised CS. Revised CS that are approved will either (1) be assigned the same CS number as the original project, if the amendment requests additional resources solely for completion of the initial project, or (2) be assigned a new CS number if the amendment proposes the addition of data/analyses to the project that will result in publication of an additional manuscript.
4. NIH / DBT / OTHER GRANT SUBMISSIONS
If an investigator plans to submit his/her CS to the National Institutes of Health (NIH), Indian Department of Biotechnology (DBT) or other funding organization as part of a grant submission (e.g., R01, R23), the CS will not undergo a typical RePORT India review and the Short CS Submittal Form should be used.
RePORT India will accept the funding institution’s judgement as to the scientific merit of the work and will not require a separate PIR in order to judge the scientific merit of the proposal. A PIR will be assigned per the process in section 1 above, however, to complete an administrative review of the CS. This will include looking for overlap with already approved work in the RePORT India Consortium and commenting on specimen and data availability. These CSs will also be assigned a Study Coordinator Reviewer, a Lab Reviewer and/or a Genetics Reviewer, as needed to examine the feasibility of implementation. Once the administrative review is complete, Steps 4, 5, and 6 in section 1 above may be skipped. Instead, the PIR must send their recommendation to the RePORT India EC Chairs who will determine if a letter of support may be provided for the purposes of the grant submission.
Projects that are submitted to DBT for NOCs need to be piloted by an Indian PI. The application needs to be submitted to DBT one month in advance for approval. All RePORT related sub-studies which relate to sample transfer or material transfer need to go through DBT.
If the funding institution decides to fund the project, no further reviews will be needed by RePORT India. If the funding institution decides not to fund the project and the investigator still wishes to proceed with the work, then the concept sheet will be assigned regular CS review to judge the scientific merit of the work (described above).
5. CO-INVESTIGATOR / CO-AUTHOR SELECTION
Approved CSs should include representation from all participating RePORT India Cohort Research Unit (CRU) sites. If this requirement is not met, the RePORT India Coordinator may then solicit co-investigators /co-authors from each of the participating CRUs. Authorship assignment will follow the existing publication policy that offers co-authorship to all sites involved in data collection.
Sites are required to propose their co-investigators/co-authors within two weeks of receiving the request from the RePORT India Coordinator. If a site does not suggest individuals in this timeframe, the site’s PI will be assigned on the project.
NOTE: If lead investigators are considering submission to journals that limit the number of coauthors, then this information and a rationale should be included in the concept sheet proposal so that details of co-authorship can be worked out in advance. In the case of re-submission to a journal with limitations on the number of authors, the journal should be approached to allow for inclusion of all co-authors and if this is not successful the issue should be discussed with the co-authors. If the journal agrees a smaller subset of key authors, followed by the phrase “for the RePORT India Consortium*”can be included; the asterisk would include a footnote referring to the appendix which has the complete list of the contributors. The inclusion of the key authors should be according to the degree of contribution to the plan/conduct of the study and preparation of the manuscript. If no resolution is reached with the co-authors, then the issue will be brought to the EC for an arbitrated decision. In principle, RePORT India supports publication in journals that acknowledge the role of large databases and collaborative research.
B. CREDIT, AUTHORSHIP, AND WRITING COMMITTEE
The following categories specify how credit and authorship is apportioned for most RePORT India projects. Special requests can be discussed and voted upon by the RePORT India publications committee by writing to Nancy Divya at reportindiacoordinator@gmail.com.
All manuscripts from approved projects are required to receive RePORT India publications committee review to ensure that all RePORT India manuscripts acknowledge that the data were collected through RePORT India. They must also credit participating institutions (RePORT India Cohort Research Unit (CRU) sites, U.S. partner institutions, supporting institutions, and funding agencies). Grant numbers should be cited when feasible. Appendix A contains a template for the RePORT India acknowledgments. All investigators must acknowledge that RePORT India specimens and data are the property of the RePORT India Consortium. Investigators are responsible for reviewing and agreeing to the RePORT India Publication Policy, ensuring that the samples and data are used in the manner outlined in the CS, and disseminating results to assigned RePORT India collaborators/co-authors in a timely manner. If an investigator later wishes to change the study methods or to expand the scope of an already approved project, then an amendment to the CS must be submitted for review by the RePORT India EC. (See Section I.A.3, above.)
The RePORT India Consortium adheres to criteria for authorship promulgated by the International Committee of Medical Journal Editors (ICMJE). All authors should be prepared to meet the following four criteria with their contributions:
- Make substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
- Draft the work or revise it critically for important intellectual content; AND
- Provide final approval of the version to be published; AND
- Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
1.
Single-Site Investigations
2.
Multi-Site Investigations
3.
Core Investigations
4.
Nested Investigations Collecting New Data
5.
Multi-Cohort Collaborative Investigations
6.
Methodological Investigations
1.
Single-Site Investigations
A single-site investigation is one using data collected from one site only and funded through that site’s RePORT India funding agreement (e.g. parent protocol) or external sources (e.g., RO1, GCRC, unobligated funds, etc.). These data may be collected as part of a pilot study, the core RePORT India common protocol, a local sub study, or be generated from local specimens collected during RePORT India Common Protocol or additional visits. For the use of RePORT India Common Protocol data, single-site investigations should be rare; investigators are encouraged to utilize the entire RePORT India Common Protocol cohorts for most projects. It is anticipated that the parent protocols will result in multiple single site publications.
Publications resulting from single-site investigations will include co-authors and other authors at the discretion of the lead investigator from the local site. Manuscripts should be approved by the site Principal Investigator prior to submission to the RePORT India Coordinator for administrative review and tracking purposes.
2.
Multi-Site Investigations
A multi-site investigation is one wherein analyses will utilize data from a subset of the RePORT India clinical sites. For these investigations, site representation will be solicited from the sites contributing data and/or analytic support.
3.
Core Investigations
A core investigation is one using data generated as part of the principal RePORT India collaborative agreements (i.e., all CRU sites and other affiliated institutions). These data may be part of the core RePORT India Common Protocol, a sub study, or generated from specimens collected as part of Common Protocol visits. Funding may come from the core collaborative agreement, RePORT India supplemental funds, other grants (e.g., RO1s), or re-apportionment of unobligated funds. The lead investigator of a core investigation does not necessarily need to be supported by RePORT India (i.e., can be an “external” investigator). However, the RePORT India reserves the right to assign a new lead author to a project if an external investigator does not wish to write up the study results but agrees that a publication is worthwhile.
Authorship for Projects Led by RePORT India Investigator
Core investigations led by a RePORT India investigator require that each CRU site Principal Investigator be offered coauthor representation in recognition of the substantial amount of operational work performed by each site for cohort recruitment, retention, data collection, and data management. After approval of the CS, the RePORT India Coordinator will email the Principal Investigators to request assignment of co-authors. Failure to name a co-author within two weeks will result in the site having the Principal Investigator serve as the co-author by default. Additional authors may also be nominated based on interest and ability to make substantial and necessary contribution to the work. All authors should meet the ICMJE criteria for authorship.
Authorship for Projects Led by “External” Investigator
Core investigations led by an external investigator also require that each CRU site Principal Investigator be offered coauthor representation. The process for assigning co-authors is the same as the process for core investigations led by RePORT India investigators. After all co-authors have been assigned, the RePORT India Coordinator will contact the external investigator with the names and email addresses for each site representative.
In addition, the lead investigator on a project should include as a co-author any investigators and analysts (RePORT India or external) that make substantial contributions to the project. All authors should meet the ICMJE criteria for authorship. If appropriate, the external investigator may take the role of senior corresponding author on the manuscript.
4.
Nested Investigations Collecting New Data
RePORT India funding through the principal collaborative agreements is limited and is necessary to support the core study protocol and scientific priorities of the RePORT India EC. Although RePORT India has limited funds for auxiliary studies, it is expected that many studies generating new data will be supported with external funds. These may include data obtained directly from participants outside of the core RePORT India Common Protocol (e.g., during interim substudy visits), specimen collection, utilization of specimens from the central biorepository, or data generated using central biorepository specimens. Nested investigations may be initiated by individuals supported or not supported on core RePORT India collaborative agreements (including RePORT India Principal Investigators). In general, the co-authorship guidelines for nested investigations will follow those for single-site, multi-site, or core investigations. Project investigators have a right to utilize and publish new data generated from external funding sources prior to sharing with other RePORT India investigators. However, once the aims of the CS have been completed and published, the new data must be transmitted to CHRD-SAS for integration with the core RePORT India Common Protocol database and released to other RePORT India investigators, upon request. This requirement may be waived for select studies by EC vote, if appropriate justification is provided.
5.
Multi-Cohort Collaborative Investigations
Proposed studies that involve pooled data from the RePORT India Common Protocol and other cohorts should include authors from each of the partner organizations involved. It is recognized that multi-cohort collaborations can result in an unwieldy number of co-authors. Hence, in general, only one or two RePORT India representatives will be assigned to multi-cohort collaborations, in addition to the project investigators. The RePORT India EC will select appropriate RePORT India co-author(s) preferably with expertise in the area of investigation. In some cases, RePORT India will be involved in multi-region collaborations as part of RePORT International. Due to the large number of possible co-authors for these collaborations, RePORT India may not always be granted the opportunity to be represented as a co-author.
6.
Methodological Investigations
Investigators may propose statistical or laboratory methodological projects that utilize data or specimens collected as part of the core RePORT India from multiple sites. The primary aim of these projects is the development or adaptation of new statistical or laboratory methods, with limited substantive results. Most of these projects are targeted towards publication in specialty journals. At the time the CS is submitted, the lead investigators may petition the RePORT India publications committee (reportindiacoordinator@gmail.com) to ask that these projects receive a single RePORT India representative for the paper.
C. REQUESTS FOR DATA, SPECIMENS, AND ANALYTIC SUPPORT
Once a project is approved by EC, the lead investigator should contact the RePORT India Coordinator, Nancy Divya for requests for data, specimens and analytic support, who in turn will forward the requests to the appropriate agency.
1.
Data Requests
2.
Specimen Requests
1.
Data Requests
All Common Protocol data requests that involve data housed at SAS or another data repository external to the site which is making the request, should be submitted to RePORT India Coordinator, Nancy Divya. The e-mail should include:
- A list of RePORT India variable names
- The visit number(s) and/or calendar dates for which data are needed
- Communication regarding the EC approval of the CS.
Variable names can be obtained from the RePORT India data elements bank. The RePORT Common Protocol data elements bank is posted on the RePORT India Web Portal and can be requested from the RePORT India Coordinator, Nancy Divya. A SAS-CHRD data programmer will be assigned to the project once a data request is made. SAS-CHRD requires a two-week time period for the production of an analytic database. If the data request is complex, additional time may be necessary.
2.
Specimen Requests
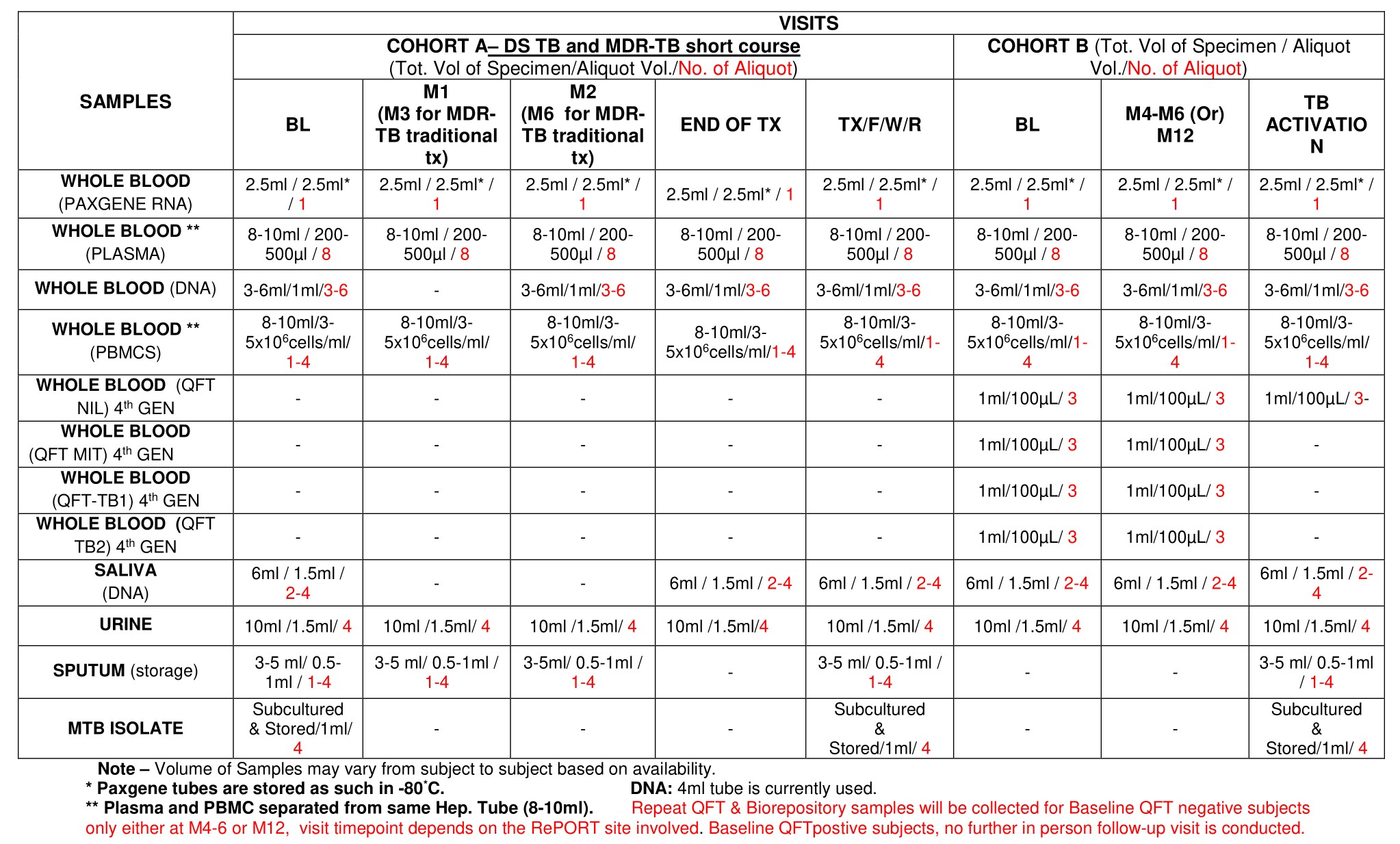
Please refer to Appendix C for a list of Common Protocol samples and storage specifications. All specimen requests should be submitted to the central repository along with the communication regarding the EC approval of the CS. Requests for specimens will not be processed until verification of local IRB/IEC approval of the Investigator AND the CRU sites from where the specimens were obtained has been provided. All requests should include a completed Central Biorepository Request Checklist or a completed DNA Biorepository Sample Request Form (for DNA requests) – both can be found at the RePORT India Web Portal and can be requested from Nancy Divya.
Selection of Specimens
If an investigator has already determined the appropriate RePORT India Participant ID (PID)-visits for his/her project, an Excel spreadsheet of RePORT India Participant IDs, visits, and visit dates should be attached to the e-mailed specimen request. If the investigator has not yet determined appropriate RePORT India Participant ID-visits, a SAS programmer will be assigned to work with the investigator to select appropriate RePORT India Participant ID-visits based on the selection criteria in the approved concept sheet.
RePORT India Specimen Allocation Committee Review
The RePORT India Specimen Allocation Committee (RISAC) is charged by the RePORT India EC to assist in the allocation of high-value RePORT India biorepository specimens. The members of the RISAC are the members of the EC and the WG chairs. The RISAC reviews all requests for the release of samples from individuals who contribute significant or unique outcomes to overall RePORT India research aims and to determine whether or not the restricted samples should be released to the requesting investigator. When necessary, the RISAC may be asked to review concept sheets that request the use of high-value samples.
High-value specimens include those from:
- Cohort B TB activation cases
- Cohort B TB activation cases enrolling in Cohort A
- Pediatric active TB cases
- TB treatment failure
- Early TB relapse
In some cases, investigators may not know whether their selected specimens will also qualify as high value samples. When high-value specimens are unexpectedly selected for an approved CS, RISAC should be notified and provide guidance concerning the request to use these specimens.
D. PROGRESS REPORTS / DEACTIVATION OF CONCEPT SHEETS
Annual progress reports (link here), beginning one year after initial concept sheet approval, will be required of all investigators with active concept sheets in order to keep a project active. These should be submitted via email to the RePORT India Coordinator. If a progress report is not completed, or if the completed progress report indicates that no activity is occurring on the project, the project will be declared inactive and designated as a “dead” project. If no progress is made on an approved concept sheet for two years, it will be automatically deactivated.
E. MANUSCRIPTS AND ABSTRACTS
The review, approval, and tracking of RePORT India publications will be handled by the RePORT India publications committee. This committee will be appointed by the RePORT India EC. The RePORT India Coordinator will serve as the administrative heads of the group and will be responsible for assuring that all of its activities are performed in a timely manner. The RePORT India Publications Committee can be contacted via email at reportindiacoordinator@gmail.com.
1.
Review of Manuscripts
2.
Review of Abstracts & Presentations
3.
Journal Submissions
4.
Submission of Manuscripts to NIH
1.
Review of Manuscripts
RePORT India Publications Committee review is mandatory for all manuscripts originating from common protocol or multiple sites. Site-specific manuscripts from parent protocol need not follow the process, however once it is accepted for presentation in conference or for publication in a journal the Publications Committee needs to be informed for archiving purposes. Co-author(s) must participate in the writing and/or review process in a timely manner. If a co-author does not participate, he or she may be removed from the manuscript at the discretion of the EC. The lead investigator should circulate a draft copy of the manuscript amongst all co-authors and incorporate co-author comments. Once the manuscript has been approved by all co-authors, the lead investigator should submit it electronically to the RePORT India Publications Committee (reportindiacoordinator@gmail.com) using the Manuscript Submittal Form. The RePORT India Coordinator will take the lead on ensuring each manuscript’s review. Manuscripts submitted without full co-author approval or with incomplete or outdated submittal forms will be returned to the author for correction before circulating. If a co-author disagrees with the main findings or methods of a manuscript, or finds the data or analysis misleading, he/she must resolve these issues with the writing group/co-authors before the manuscript is submitted to the publications committee. If a co-author still finds fault with the version submitted to the publications committee, he or she should address these concerns with the lead investigator. The co-author may also indicate his or her concerns by circulating their comments. If one or more of the co-authors still disagree with the lead author regarding analyses in the paper, he or she may wish to be removed as a co-author. The submitting investigator should document any change in authorship at the time of manuscript submittal to the publications committee. The publications committee is responsible for ensuring that administrative requirements such as co-author reviews and approvals, and consortium acknowledgements are present. They are also responsible for tracking consortium publications. They are not responsible for reviewing the scientific content of the manuscript. Ensuring scientific integrity and quality of content is the responsibility of each writing team.
2.
Review of Abstracts & Presentations
Final abstracts and presentations must adhere to the following guidelines:
- Abstracts must be associated with an EC-approved concept sheet (single site investigations such as parent protocol abstracts are exempt from this requirement).
- Co-authors should be the same as the ones assigned for the EC-approved concept sheet. If an investigator needs the list of assigned co-authors, he/she can contact the RePORT India publications committee at reportindiacoordinator@gmail.com.
- RePORT India-wide abstracts require co-authors from each RePORT India CRU site.
- Abstracts must be provided to co-authors before the abstract is submitted to the publications committee for review and approval. Co-authors must be given at least at least five business days to review and approve the abstract before it is submitted for publications committee review and approval. If all co-authors are in agreement, this requirement can be waived. The submitting investigator should indicate upon submittal to the publications committee that co-authors were provided five business days to review and approve or unanimously agreed to waive this rule.
- If a co-author does not respond within the five business days period, the submitting investigator can assume approval and proceed with submission to the publications committee for review and approval. If a co-author wishes to be removed from the abstract, the submitting investigator should indicate this upon submittal to the publications committee.
- All abstracts should be submitted to the RePORT India publications committee for tracking purposes, to verify compliance with the RePORT abstract guidelines, and to prioritize abstract submissions to conferences that limit abstract submission numbers.
If the aforementioned guidelines are not met, the following policy will take effect:
- If an abstract is submitted to the RePORT India publications committee without co-author approval, the abstract will be returned to the lead investigator for circulation to all co-authors for review and approval.
- If the abstract is being submitted to a conference that limits the number of abstracts that can be submitted from any one cohort, the following abstract submission policy will take effect: All abstracts must be submitted to co-authors for review and approval at least five business days before submitting for publications committee review.
- All co-author approved abstracts must be submitted for publications committee review at least five business days before the abstract submission deadline.
- After all compliant abstracts have been received, they will be distributed to publications committee members and members will be asked to apply a forced ranking system to rank the top six (or the number set by the conference) abstracts. Investigators from the top abstracts will be notified that they can submit to the conference. All others will be informed that their abstract was not approved for submission.
- All other abstracts submission guidelines apply.
Investigators must email their proposed abstract to the publications committee at reportindiacoordinator@gmail.com at least at least five business days prior to the abstract submission deadline. The following information must be included with the submission:
- Name of conference
- Dates of conference
- Conference abstract submission deadline
- Abstract title
- CS# (or Protocol Name if no CS number)
- List of co-authors
- Copy of the abstract
- Verification that all co-authors reviewed and approved the abstract
- Description of conference’s limit (if any) on the number of abstracts submitted from one cohort
The publications committee will verify that the conditions are met.
3.
Journal Submissions
The lead investigator must notify the RePORT India publications committee electronically (reportindiacoordinator@gmail.com), using the Manuscript Submittal Form whenever a manuscript is accepted to be published in a journal. The lead investigator should update the Publication Submission Form and resend to the RePORT India publications committee. This form helps the consortium track all RePORT India publications and ensures publications are properly archived and listed in the publication list. If a manuscript is accepted for publication, lead authors are also responsible for sending a PDF (Portable Document Format) version of the published article to the RePORT India publications committee electronically (reportindiacoordinator@gmail.com).
Please remember that presentations or manuscript submissions that do not have prior publications committee approval and NIH and DBT notification are inconsistent with the spirit of collaborative research. Disregard of this policy may result in future denial of access to RePORT India data and a cessation of collaborative support. In addition, presentation or submission of unapproved manuscripts puts the investigator at risk of disciplinary proceedings by the RePORT India EC and/or funding agencies.
Publications and presentations shall be in compliance with the rules and procedures of the disclosure set forth in the Privacy Act. Confidential or proprietary information shall not be disclosed without the prior written consent of the individual or institution. Privacy Act compliance and documentation of written disclosure consents are the responsibility of each institution involved in the paper/presentation.
4.
Submission of Manuscripts to NIH
NIH requires all RePORT India investigators who are participating in this study, which is funded by NIH, to make their peer-reviewed final manuscripts available to other researchers and to the public at the National Library of Medicine’s (NLM) PubMed Central (PMC) within 12 months of the publication date. The RePORT India Coordinator will assist with this process. NIH expects investigators to submit an electronic copy of the final version of the manuscript accepted for publication. A separate submission is not necessary if the manuscript has been accepted by a journal that permits free access to PDFs within 12 months of publication. (These journals are listed at the above web site.) To submit PDFs of articles please visit the NIHMS system web site.
Publicity Policy
A. LOCAL PUBLICITY
Local publicity refers to media distributed to each site’s city, metropolitan area, or state. This includes local TV stations, radio stations, and newspapers; city, county, or state health department newsletters; hospital publications; and local university publications, not available by general public subscription.
- Each site may release general information about their site to local media. If possible, communications should be cleared in advance by the EC and funding agencies.
- Study data or published analyses should never be disclosed without prior clearance by the EC and funding agencies.
B. REGIONAL / NATIONAL / INTERNATIONAL PUBLICITY
National/international publicity refers to media distributed widely outside each site’s city, metropolitan area, state, or country. This includes network television, network radio, major newspapers, national newsletters and widely disseminated university publications. Because national/international publicity may impact the overall reputation of the RePORT India Consortium, all questions by national/international media should be directed to the site’s Principal Investigator, who should then notify DBT, ICMR and the EC.
C. GENERAL GUIDELINES
- If significant questions arise about RePORT India CRU Sites or funding agencies (e.g., “How much is XX agency spending overall on the RePORT India study?”), refer the reporter to the appropriate agency (i.e., investigators at those sites or agencies).
- When answering questions, make clear distinctions between personal opinions and positions that have been arrived at jointly by the RePORT India collaborators.
Appendix A
RePORT India Acknowledgments
Data in this manuscript were collected as part of the Regional Prospective Observational Research for Tuberculosis (RePORT) India Consortium. This project has been funded in whole or in part with Federal funds from the Government of India’s (GOI) Department of Biotechnology (DBT), the Indian Council of Medical Research (ICMR), the United States National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID), Office of AIDS Research (OAR), and distributed in part by CRDF Global. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the DBT, the ICMR, the NIH, or CRDF Global. Any mention of trade names, commercial projects, or organizations does not imply endorsement by any of the sponsoring organizations.
Participating Institutions, Principal investigators, co-principal investigators, DBT, ICMR, and NIAID staff include:
Byramjee Jeejeebhoy Government Medical College (BJGMC): Vidya Mave, Samir Joshi | National Institute for Research in Tuberculosis (NIRT): Padmapriyadarsini Chandrasekaran | Johns Hopkins University (JHU): Amita Gupta | Hinduja Hospital: Zarir F. Udwadia, Camilla Rodrigues, Tester F. Ashavaid | Bhagawan Mahavir Medical Research Centre (BMMRC): Vijaya Valluri | University of Texas Health Center: Ramakrishna Vankayalapati | Christian Medical College, Vellore (CMC): Devasahayam Christopher | University of Cambridge: Lalita Ramakrishnan | Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER): Gautam Roy; | Boston Medical Center: Natasha Hochberg | Rutgers: Jerrold Ellner | New Jersey Medical School: Padmini Salgame | M.V. Diabetes Research Centre (MVDRC): Vijay Viswanathan | University of Massachusetts Medical School: Hardy Kornfeld | Centre for Health Research and Development – Society for Applied Studies (CHRD-SAS): Sunita Taneja | Pharmaceutical Product Development, Inc. (PPDI): Bambra Stokes, Kyle Foster | FHI 360: Kimberly Booher | DBT: Jyoti Logani; | ICMR: Rashmi Arora | NIAID: Roxana Rustomjee | CRDF Global: Chris Maxwell.
Appendix B
Available Concept Sheet and Manuscript Reviewers (area of expertise)
PI REVEIWERS (PIR)
- Vidya Mave
- Padmapriyadarsini Chandrasekaran
- Amita Gupta
- Vijaya Valluri
- Ramakrishna Vankayalapati
- Devasahayam Christopher
- Lalita Ramakrishnan
- Gautam Roy
- Jerrold Ellner
- Vijay Viswanathan
- Samir Joshi
- Hardy Kornfeld
- Natasha Hochberg
- Padmini Salgame
- Zarir F. Udwadia
- Camilla Rodrigues
- Tester F. Ashavaid
WORKING GROUP CHAIRS (BACK-UP PIR)
- Padmini Salgame
- Ramakrishna Vankayalapati
- Sonali Sarkar
- Padmapriyadarsini Chandrasekaran
STUDY COORDINATOR REVIEWERS (SCR)
- Mandar Paradhkar
- Deepa Shankar
- Prudhula Kamakshi
- Krithikaa Sekar
- Reshma A
- Shruthi BS
- Ishita Gajjar
LAB REVIEWERS (LR)
- Luke Elizabeth Hanna
- Amsaveni Sivaprakasam
- Vandana Kulkarni
GENETICS REVIEWERS (GR)
- TBD
Appendix C
RePORT India Central Biorepository Specimen List & Storage Specifications (Common Protocol)
Sumbittal Form for Internal Investigators
All form fields are compulsory. Enter NA if the field is Not Applicable. Please go through the entire contents of the form before you begin. The form cannot be saved halfway through the submission. If you prefer to fill the form offline, please download it and email the completed form to RePORT India Coordinator, U.S. Secretariat and Executive Committee Chairs at dgnanadason@crdfglobal.org
Submittal Form For External Investigators
submittal form for external investigators
